Good news at the Spring Festival! Denture Base, the photosensitive resin independently researched, developed and produced by Riton has successfully passed the review of U.S. Food and Drug Administration, and got the FDA certification!

U.S. Food and Drug Administration is the highest law enforcement agency authorized by the US Congress, the federal government to specialize in food and drug administration. FDA holds an essential role in the regulation of food, drug, medical device, cosmetics, animal food and medicine and other fields in the world, and its strict standards ensure the safety and validity of products. For enterprises, getting a FDA certification not only means a stepping stone into the US market, but also a symbol of the product’s excellent quality.
The latest FDA-approved Denture Base is one of the products in Riton photosensitive resin consumable item series.

This series of materials are developed and produced by Riton, are especially created for SLA technique in dentistry, and can be widely used for printing and production of multiple kinds of dental resin models. On the basis of ensuring high printing success rate, they can give the finished products outstanding precision, excellent stability and good wear resistance.
Denture Base is a resin material that used for partial or full mouth base production in the series. Denture base is the bracket part for arranging artificial teeth and evenly transmitting the force borne by artificial teeth to the oral tissue in the repairing process of dentition defect.
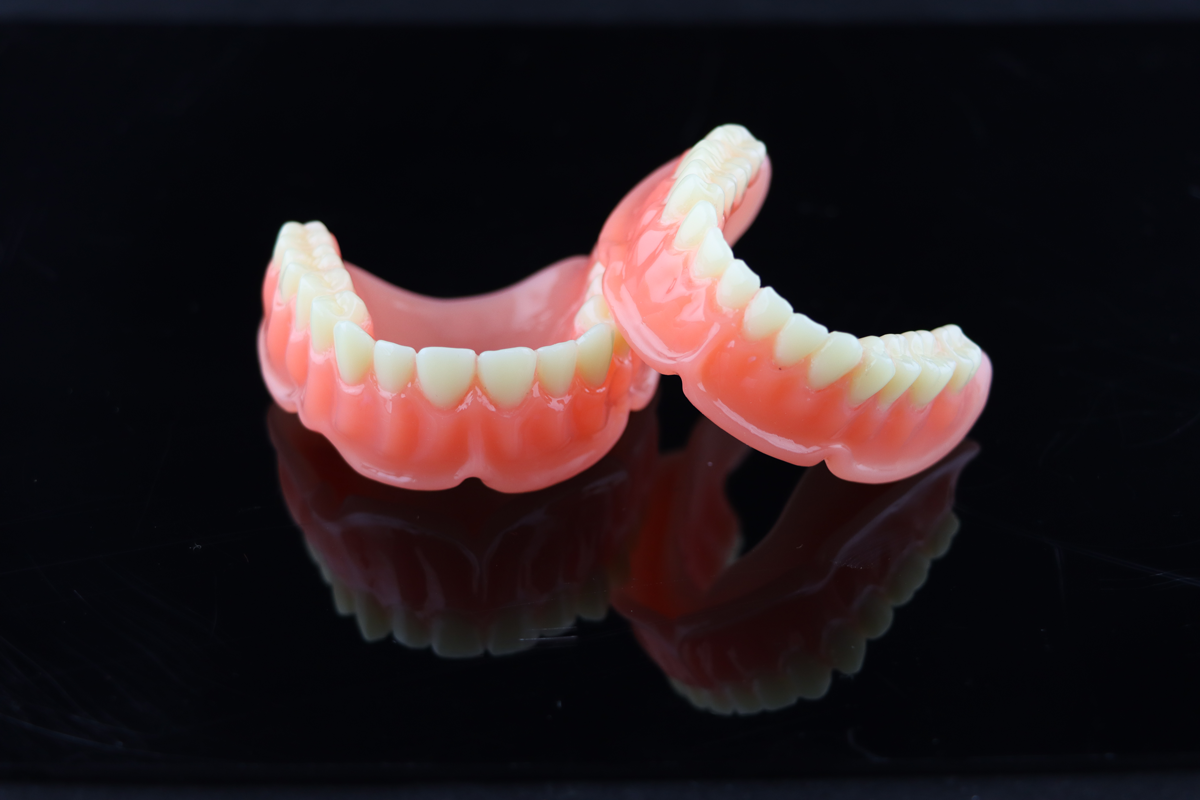
The base printed by the product is safe and odorless. It has high strength, bending, wear resisting features and excellent biocompatibility. Its mechanical performance and water absorption both meet the requirements of dental prosthetics. The surface of finished products will show natural textures and colors after simple polishing, which achieves the balance and integration of practicability and aesthetics.
Denture Base Parameters

Color: light pink
Specification: 1kg per bottle
Wavelength: 405nm
Suiting Device: RXDent-L230
Application Scope: mobile prosthetics
Material Certification: NMPA, FDA
Testing Data
Test Item | Unit | Test Standard | Numerical Value |
Bending Strength | Mpa | ASTM D790 | 90 |
Bending Modulus | Mpa | ASTM D790 | 1860 |
Hardness | Shore D | ASTM D2240 | 86 |
Notched Impact Strength | J/m | ASTM D648 | 65 |
Sample Display
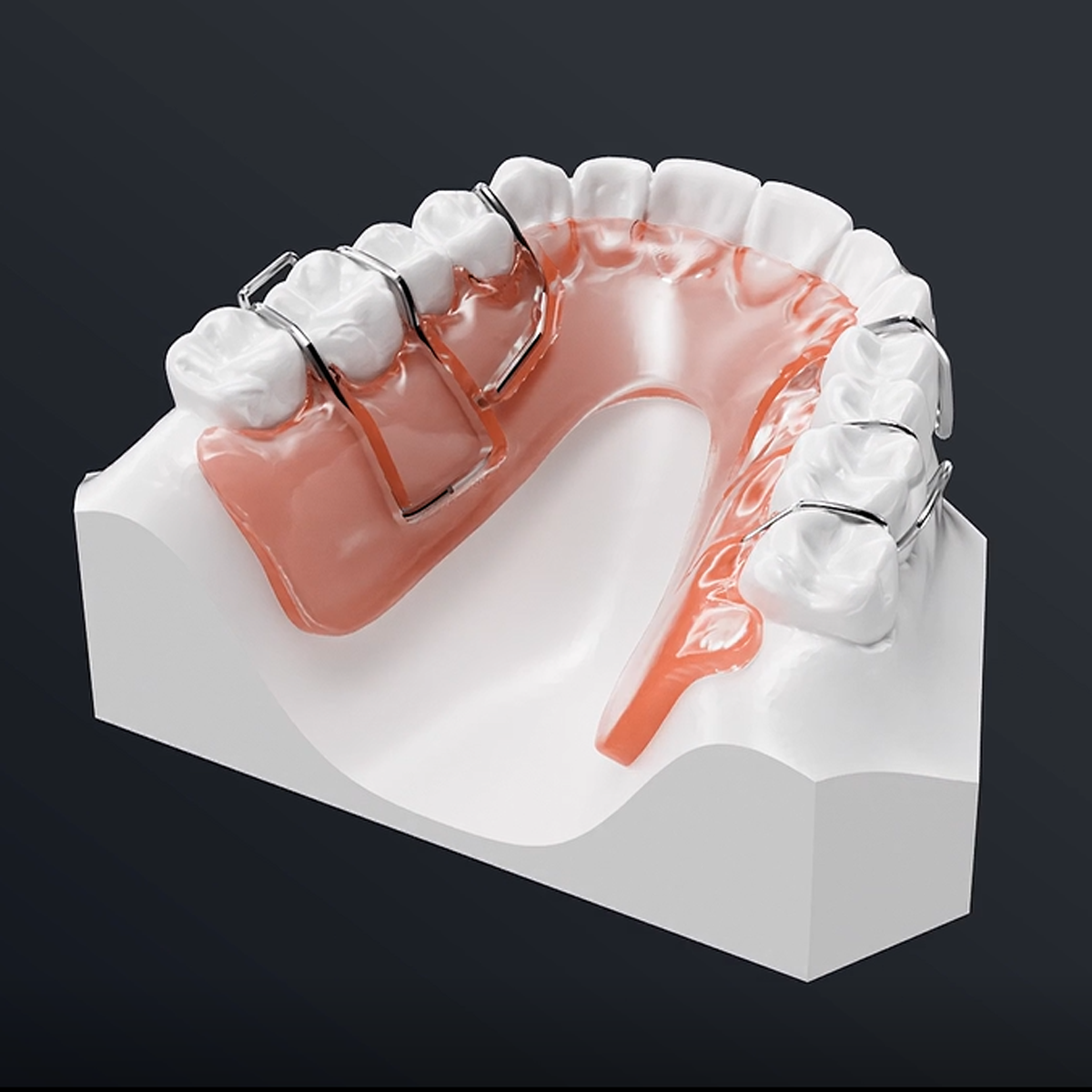
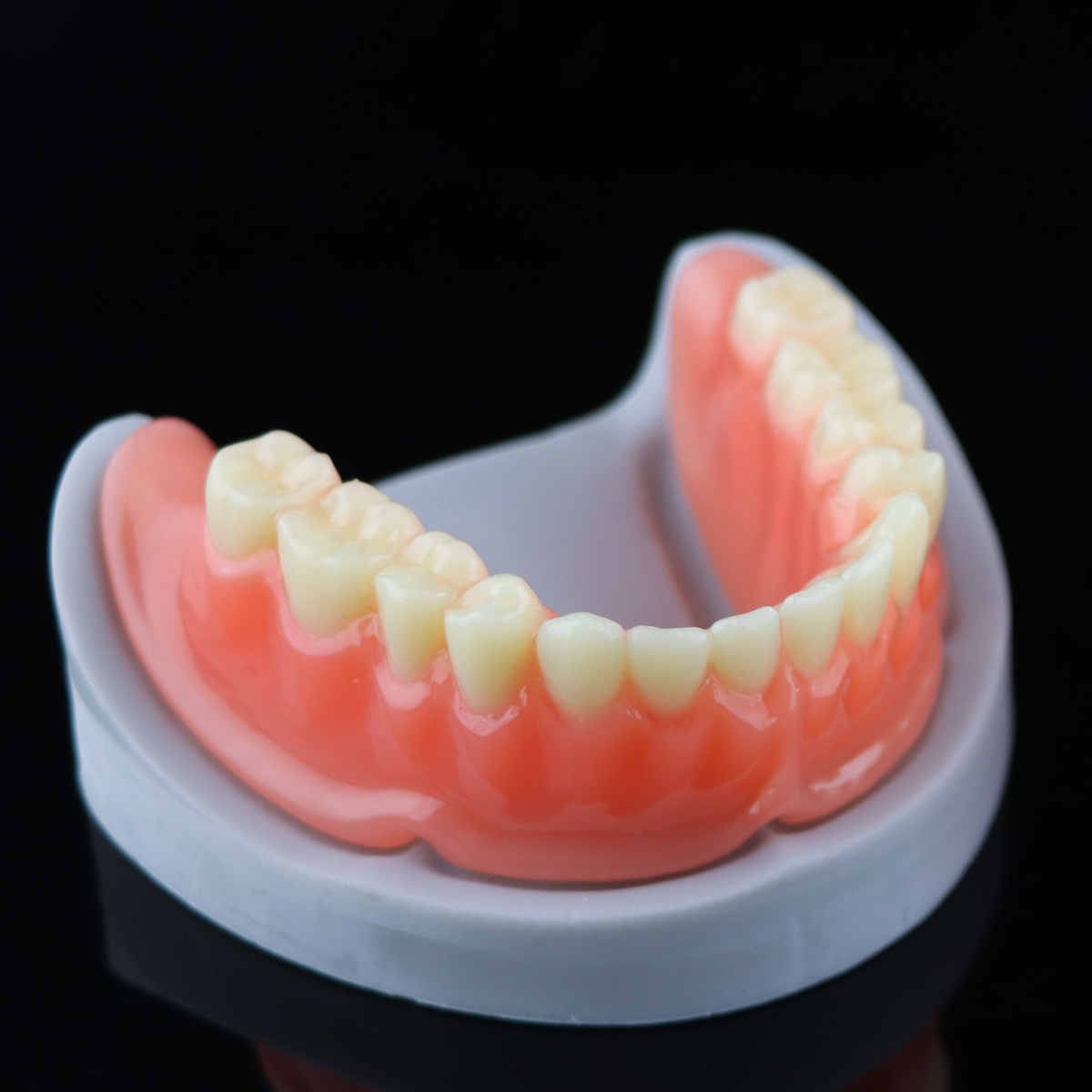
By now, Riton already has many photosensitive resins that have got the NMPA, FDA and other certification from authorities at home and abroad, provides more diverse and high-quality choices of consumable items for dental photosensitive additive manufacturing.
In recent years, under the impact of the increase of all people’s oral health awareness and income, the need for dentures keeps increasing around the world, the market scale of oral prosthesis material industry has been increasing.
Data shows that the market scale of oral prosthesis material industry in China has increased from 14.858 billion RMB in 2018 to 22.216 RMB in 2022. Professional institution predicts that by 2025, the market scale of oral prosthesis material industry will arrive 30.333 billion RMB with a composition annual rate of growth of 10.0%. Moreover, the market scale in China will arrive 48.492 billion RMB by 2029.
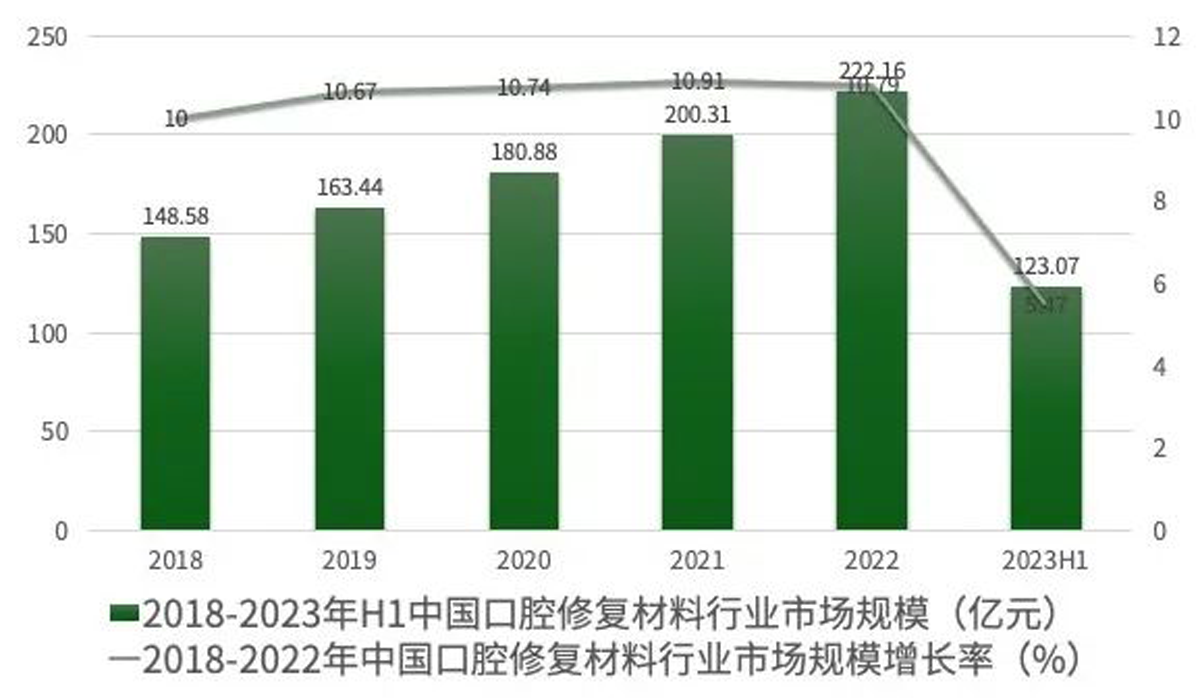
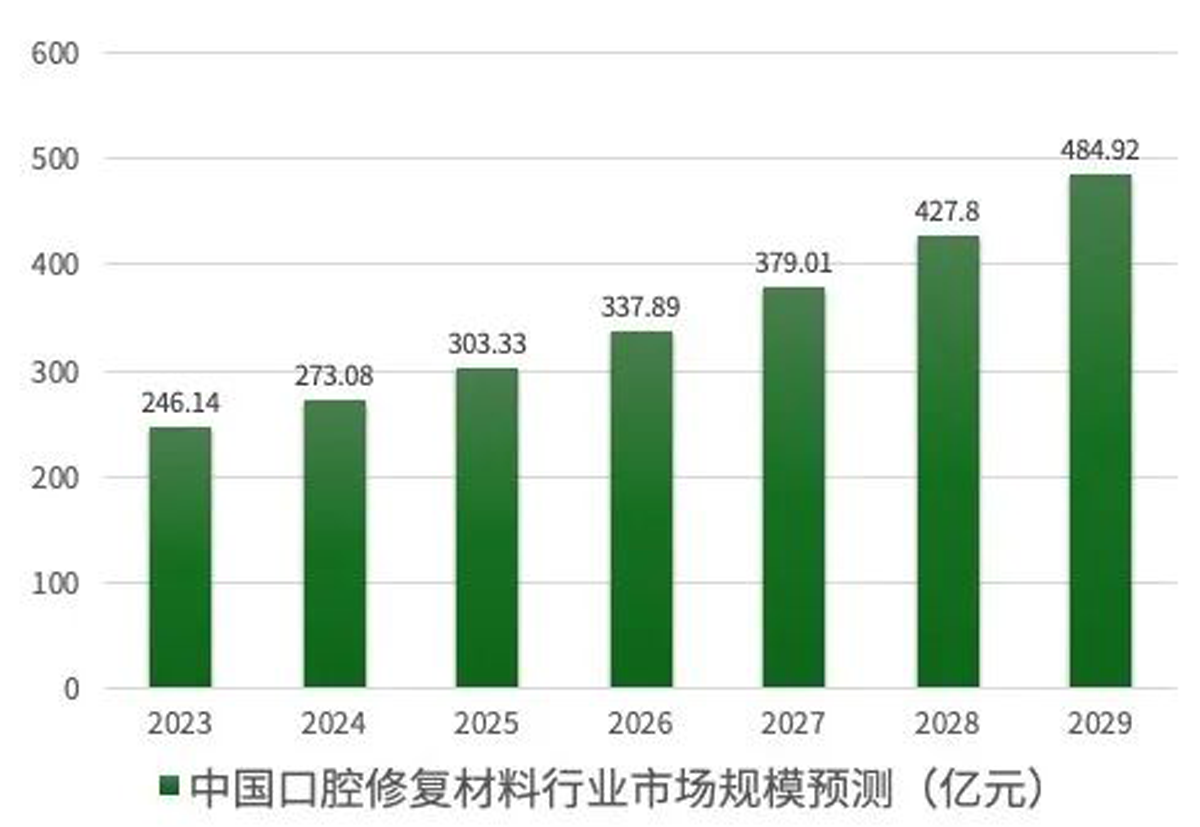
Data Source: Zhiyanzhan Industrial Research Institute
Look into the future, with the increasing aging phenomenon around the world, the need in denture market will have a further lift, and this trend will lead the increase of need for oral prosthesis material in the same pace.
Riton prospectively arranged the aspect of oral prosthesis material with its years of experience and keen insight in dentistry.
In 2021, Riton 3D Technology Co., Ltd, a wholly owned subsidiary of Riton was found, and it’s specialized in R&D of dental metal materials, dental resin 3D printing devices, resin materials and expansion of digital design services.

Nowadays, Riton 3D Technology has already made many achievements. Not only have many of its materials successfully passed a series of certifications such as CE(EU), ROHS, FCC(North America), IC, Class Ⅲ Medical Devices and so on, its excellent quality of products and services also have helped it successfully establish deep cooperation relationships with more than 350 dental laboratories around the world, meanwhile provide digital design training for more than 60 dental laboratories. It provides over 1,000 digital designs every month and sells more than five tons of consumable items per month, becomes a trusted choice in the industry.

Getting the FDA certification for the photosensitive resin this time is a highly recognition from global authorities to Riton’s R&D ability and product quality. In the future, Riton will keep striving, keep the professionalism and continuously explore and break edges in denture 3D printing field under the base of product innovation and the core of customer experience, provide more high-quality equipment, consumables and services for the industry and customers, contribute our strength to promote the development of new and good productivity!
Riton 3D Printers
